
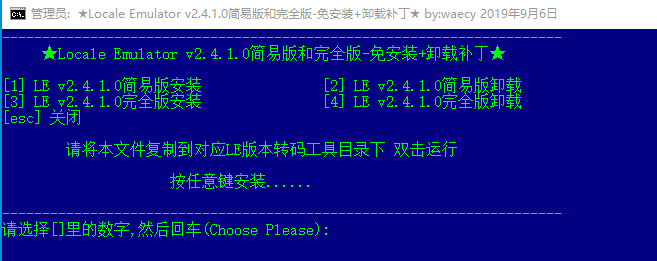
- #Vnr locale emulator pdf
- #Vnr locale emulator update
- #Vnr locale emulator verification
- #Vnr locale emulator code
Looking at the data, the year 2016 was very similar to 2015. The annually published Vnr statistics document is soon to be published here in VnrWiki. The Vnr statistics document 2016 is published here in VnrWiki. Norway has also prepared a document Implementation of 2D data matrix in Norway.īoth documents are intended to help the pharma companies when planning the transition from NTIN to GTIN and the serialisation process in relation to the FMD. The document The serialisation of Nordic packages, country specific requirements was first prepared by LIF in Sweden and then revised in co-operation with the other Nordic countries. Two new documents are published under the section Transition from NTIN to GTIN. Section 5.7 made clearer and the notification regarding the need of different GTINs added.
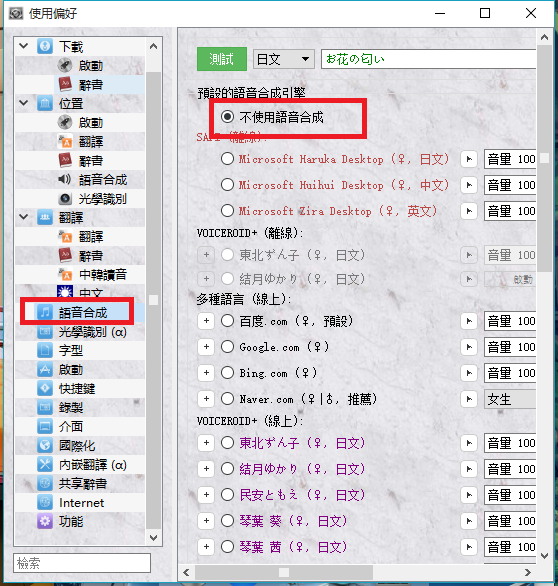
#Vnr locale emulator update
The example for the EXP date in the EEA licence plate picture has been amended.Ī small update in the Nordic Article Number instruction booklet. The document The serialisation of Nordic packages, country specific requirements has been updated.
#Vnr locale emulator code
The use of the terms barcode and product code has also been standardized. Norway will be able to handle more than one product code to each Vnr from 2018. The country specific information for Norway has been updated.
#Vnr locale emulator pdf
The pdf on From NTIN to GTIN has been updated accordingly. The Norwegian Medicines Agency has now agreed to keeping both linear and 2D code on the packages after Feb 2019. The Vnr statistics 2017 document has been published.Ī pdf prepared by DMVO in Denmark, has been added under the section From NTIN to GTIN. Vnrs can now be ordered for all herbal medicines in the other Nordic countries, but Denmark.Ī new version of the Nordic Vnr guidelines is published.
#Vnr locale emulator verification
A new type of notification has been introduced in Finland, a Medicines Verification notification.Ī new version of the Nordic Vnr guidelines is published. The country specific information for Finland has changed. The email address in Sweden, for receiving more information regarding the eVerification, is now document The serialisation of Nordic packages, country specific requirements has been updated with some small adjustments concerning Denmark. The documents in VnrWiki, related to the FMD, are being updated andĪ new document Linkage between Vnrs and GTINs in the Nordic countries has been added under the section From NTIN to GTINĪ small update has been made to the document The serialisation of Nordic packages, country specific requirements. The country specific information for Finland (pdf) The serialisation of Nordic packages -country specific requirements (pdf) The following documents have been updated in VnrWiki: The document Vnr statistics 2018 has been added under Vnr statistics. The document Vnr statistics 2019 has been added under Vnr statistics. This guideline concerns only human medicines. – with “readability needs to be ensured” is meant the machine readability of the data carrierĪ new guideline regarding identifiers on single packages inside multi packages has been added. – the GTIN can be identical on the primary pack and the secondary pack, IF the pack size is 1:1 (picture 2.) – the guideline is a recommendation, not a requirement – the guideline concerns only human medicines The information regarding the Norwegian Vnr pricing was added.Īn updated version of the guideline Action plan: The need for standard identifiers on human primary packages in the Nordics, Primary package identifiers_summary OnePager ( pdf ) A summary of the guideline regarding primary package identifiers was added


 0 kommentar(er)
0 kommentar(er)
